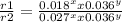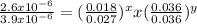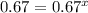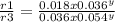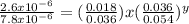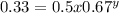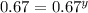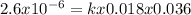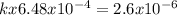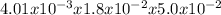Consider the reaction of peroxydisulfate ion (s2o2−8) with iodide ion (i−) in aqueous solution: s2o2−8(aq)+3i−(aq)→2so2−4(aq)+i−3(a q). at a particular temperature the rate of disappearance of s2o2−8 varies with reactant concentrations in the following manner: experiment s2o2−8(m) i−(m) initial rate (m/s) 1 0.018 0.036 2.6×10−6 2 0.027 0.036 3.9×10−6 3 0.036 0.054 7.8×10−6 4 0.050 0.072 1.4×10−5 what is the rate of disappearance of i− when [s2o2−8]= 1.8×10−2 m and [i−]= 5.0×10−2 m ?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1


Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
Consider the reaction of peroxydisulfate ion (s2o2−8) with iodide ion (i−) in aqueous solution: s2o...
Questions



Mathematics, 06.10.2020 22:01


English, 06.10.2020 22:01


Mathematics, 06.10.2020 22:01

Mathematics, 06.10.2020 22:01

Mathematics, 06.10.2020 22:01


History, 06.10.2020 22:01




Mathematics, 06.10.2020 22:01





Social Studies, 06.10.2020 22:01

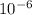 M/s
M/s![[S2O2^{-8} ]^{x} x [I^{-} ]^{y}](/tpl/images/0227/6705/d4b54.png)