
Chemistry, 12.09.2019 01:10 maehardy4134
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.60g of sodium carbonate is mixed with one containing 5.14 of silver nitrate.
1.) how many grams of sodium carbonate are present after the reaction is complete?
2.)how many grams of silver nitrate are present after the reaction is complete?
3.)how many grams of silver carbonate are present after the reaction is complete?
4.) how many grams of sodium nitrate are present after the reaction is complete?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3

Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1
You know the right answer?
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution...
Questions





Arts, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10


Mathematics, 12.12.2020 16:10


Mathematics, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10





Engineering, 12.12.2020 16:10

Biology, 12.12.2020 16:10

English, 12.12.2020 16:10

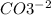 , and the ion nitrate is
, and the ion nitrate is 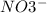 .
. 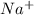 . Silver has only one electron too, so the cation will be
. Silver has only one electron too, so the cation will be 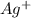 .
. 

