Chemistry, 12.09.2019 05:30 amakayla57
An unknown compound, x is thought to have a carboxyl group with a pka of 2.0 and another ionizable group with a pka between 5 and 8. when 75 ml of 0.1 m naoh was added to 100ml of a 0.1 m solution of x at ph 2.0, the ph increased to 6.72. calculate the pka of the second group of x.
source

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1
You know the right answer?
An unknown compound, x is thought to have a carboxyl group with a pka of 2.0 and another ionizable g...
Questions



Biology, 22.06.2019 17:00


Health, 22.06.2019 17:00



Biology, 22.06.2019 17:00



Mathematics, 22.06.2019 17:00





Mathematics, 22.06.2019 17:00


Mathematics, 22.06.2019 17:00


History, 22.06.2019 17:00

![pH = -log[H^{+}]](/tpl/images/0228/7557/8d3ec.png) , and pKa = -logKa. Ka is the equilibrium constant of the acid.
, and pKa = -logKa. Ka is the equilibrium constant of the acid. ![pH = pKa - log \frac{[HA]}{[A^{-}]}](/tpl/images/0228/7557/93c05.png)
![[A^{-}]](/tpl/images/0228/7557/fe74d.png) is the concentration of the anion which forms the acid.
is the concentration of the anion which forms the acid.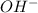 will be
will be