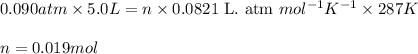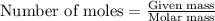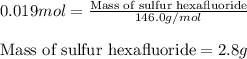Sulfur hexafluoride gas is collected at 14.0°c in an evacuated flask with a measured volume of 5.0l. when all the gas has been collected, the pressure in the flask is measured to be 0.090atm . calculate the mass and number of moles of sulfur hexafluoride gas that were collected. be sure your answer has the correct number of significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
Sulfur hexafluoride gas is collected at 14.0°c in an evacuated flask with a measured volume of 5.0l....
Questions





History, 03.02.2020 16:43



Biology, 03.02.2020 16:43

Chemistry, 03.02.2020 16:43



Physics, 03.02.2020 16:43

Mathematics, 03.02.2020 16:43


Mathematics, 03.02.2020 16:43

Mathematics, 03.02.2020 16:43



Mathematics, 03.02.2020 16:43

Social Studies, 03.02.2020 16:43

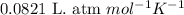
![14^oC=[273+14]K=287K](/tpl/images/0229/0798/930e3.png)