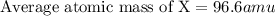An imaginary element (x) on mars is composed of three isotopes, 10.68% of isotope x-95 with a mass of 95.0 amu, 16.90% of isotope x-96 with a mass of 96.0 amu, and 72.42% of isotope x-97 with a mass of 97.0 amu. calculate the atomic mass (in amu) of the element. type in your answer with 3 significant figures.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1

Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1
You know the right answer?
An imaginary element (x) on mars is composed of three isotopes, 10.68% of isotope x-95 with a mass o...
Questions

Computers and Technology, 07.05.2021 16:50



English, 07.05.2021 16:50

Geography, 07.05.2021 16:50

Mathematics, 07.05.2021 16:50


English, 07.05.2021 16:50

Mathematics, 07.05.2021 16:50


Computers and Technology, 07.05.2021 16:50




Mathematics, 07.05.2021 16:50


Physics, 07.05.2021 16:50


Mathematics, 07.05.2021 16:50

Mathematics, 07.05.2021 16:50

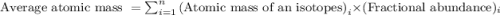 .....(1)
.....(1)![\text{Average atomic mass of X}=[(95\times 0.1068)+(96\times 0.1690)+(97\times 0.7242)]](/tpl/images/0229/1284/93747.png)