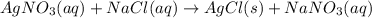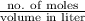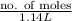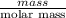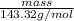Chemistry, 12.09.2019 20:30 TerronRice
When solutions of silver nitrate and sodium chloride are mixed, a precipitation reaction occurs. what mass of precipitate can be produced from 1.14 l of a 0.269 m solution of silver nitrate reacting with excess sodium chloride?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3

Chemistry, 23.06.2019 14:00
Which is not true regarding reaction rates? (2 points) catalysts are not used up in the reaction. catalysts speed up reactions by lowering the activation energy. reaction rates decrease as the concentration of reactants decrease. during reactions, concentrations of all reactants decrease at the same rate.
Answers: 1
You know the right answer?
When solutions of silver nitrate and sodium chloride are mixed, a precipitation reaction occurs. wha...
Questions

Mathematics, 22.03.2021 05:00


Mathematics, 22.03.2021 05:00



Health, 22.03.2021 05:00

Mathematics, 22.03.2021 05:00



Mathematics, 22.03.2021 05:00



Social Studies, 22.03.2021 05:00






Computers and Technology, 22.03.2021 05:00