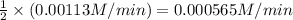Chemistry, 12.09.2019 20:30 owlgirl554
The rate of decomposition of n2o5 in ccl4 at 317 k has been studied by monitoring the concentration of n2o5 in the solution. 2 n2o5(g) → 4 no2(g) + o2(g) initially the concentration of n2o5 is 2.36 m. at 177 minutes, the concentration of n2o5 is reduced to 2.16 m. calculate the average rate of this reaction in m/min.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:40
Achemistry student weighs out of phosphoric acid , a triprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. calculate the volume of solution the student will need to add to reach the final equivalence point. round your answer to significant digits.
Answers: 3

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2
You know the right answer?
The rate of decomposition of n2o5 in ccl4 at 317 k has been studied by monitoring the concentration...
Questions



Mathematics, 10.03.2020 06:51














History, 10.03.2020 06:52



![-\frac{1}{2}\frac{[N_{2}O_{5}]}{\Delta t}=\frac{1}{4}\frac{\Delta [NO_{2}]}{\Delta t}=\frac{\Delta [O_{2}]}{\Delta t}](/tpl/images/0229/1014/2f810.png)
![-\frac{1}{2}\frac{[N_{2}O_{5}]}{\Delta t}](/tpl/images/0229/1014/bf936.png) represents average rate of disappearance of
represents average rate of disappearance of 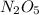 ,
, ![\frac{1}{4}\frac{[NO_{2}]}{\Delta t}](/tpl/images/0229/1014/70f91.png) represents average rate of appearance of
represents average rate of appearance of 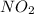 and
and ![\frac{[O_{2}]}{\Delta t}](/tpl/images/0229/1014/eef79.png) represents average rate of appearance of
represents average rate of appearance of 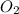
![-\frac{[N_{2}O_{5}]}{\Delta t}](/tpl/images/0229/1014/beb02.png) =
= 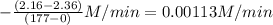
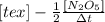 " alt="-\frac{1}{2}\frac{[N_{2}O_{5}]}{\Delta t}" />" /> =
" alt="-\frac{1}{2}\frac{[N_{2}O_{5}]}{\Delta t}" />" /> =