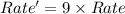Consider the following reaction: 2 no(g) + 2h2(g) → n2(g) + 2 h2o(g) the rate law for this reaction is first order in h2 and second order in no. what would happen to the rate if the initial concentration of no tripled while all other factors stayed the same? the rate will increase by a factor of 9. the rate will decrease by a factor of 3. the rate will double. the rate will triple. the rate will remain constant.

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 23.06.2019 06:30
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3
You know the right answer?
Consider the following reaction: 2 no(g) + 2h2(g) → n2(g) + 2 h2o(g) the rate law for this reaction...
Questions


Mathematics, 22.04.2021 17:50



Mathematics, 22.04.2021 17:50


Mathematics, 22.04.2021 17:50







Mathematics, 22.04.2021 17:50


Mathematics, 22.04.2021 17:50

History, 22.04.2021 17:50

Physics, 22.04.2021 17:50

Mathematics, 22.04.2021 17:50

Mathematics, 22.04.2021 17:50

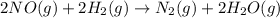
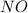 = 2
= 2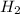 = 1
= 1![Rate=k[NO]^2[H_2]^1](/tpl/images/0229/1480/39530.png)
![Rate'=k[3\times NO]^2[H_2]^1](/tpl/images/0229/1480/e80a2.png)
![Rate'=k[3]^2[NO]^2[H_2]^1](/tpl/images/0229/1480/b88df.png)
![Rate'=k\times 9[NO]^2[H_2]^1](/tpl/images/0229/1480/d2dcb.png)