
Chemistry, 12.09.2019 21:20 seaotter7140
As a technician in a large pharmaceutical research firm, you need to produce 350. ml of 1.00 m potassium phosphate buffer solution of ph = 7.07. the pka of h2po4− is 7.21. you have the following supplies: 2.00 l of 1.00 m kh2po4 stock solution, 1.50 l of 1.00 m k2hpo4 stock solution, and a carboy of pure distilled h2o. how much 1.00 m kh2po4 will you need to make this solution?

Answers: 2
Another question on Chemistry



Chemistry, 23.06.2019 04:40
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1

Chemistry, 23.06.2019 05:50
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1
You know the right answer?
As a technician in a large pharmaceutical research firm, you need to produce 350. ml of 1.00 m potas...
Questions

Mathematics, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

Spanish, 17.09.2020 16:01

English, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

Chemistry, 17.09.2020 16:01

Business, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

History, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

French, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

Mathematics, 17.09.2020 16:01

Spanish, 17.09.2020 16:01

![\frac{[HPO4^{2-}] }{[H2PO4^{-}]}](/tpl/images/0229/1588/94f69.png)
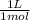 = 0,203 L ≡ 203 mL of 1,00M KH₂PO₄
= 0,203 L ≡ 203 mL of 1,00M KH₂PO₄

