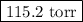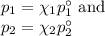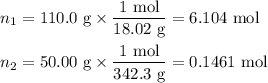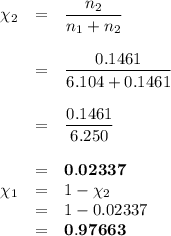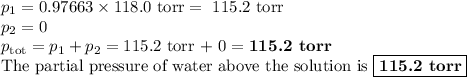Chemistry, 12.09.2019 21:30 pegflans314
Asolution is prepared by adding 50.00 g of lactose (milk sugar) to 110.0 g of water at 55 °c. the partial pressure of water above the solution is torr. the vapor pressure of pure water at 55 °c is 118.0 torr. the mw of lactose is 342.3 g/mol. a solution is prepared by adding 50.00 g of lactose (milk sugar) to 110.0 g of water at 55 °c. the partial pressure of water above the solution is torr. the vapor pressure of pure water at 55 °c is 118.0 torr. the mw of lactose is 342.3 g/mol. 156.8 2.757 115.2 282.3 81.1

Answers: 3
Another question on Chemistry


Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 06:20
Type the correct answer in each box.balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1
You know the right answer?
Asolution is prepared by adding 50.00 g of lactose (milk sugar) to 110.0 g of water at 55 °c. the pa...
Questions


Mathematics, 21.11.2020 02:30

English, 21.11.2020 02:30



SAT, 21.11.2020 02:30


Mathematics, 21.11.2020 02:30

Geography, 21.11.2020 02:30

Geography, 21.11.2020 02:30

French, 21.11.2020 02:30

Social Studies, 21.11.2020 02:30



Biology, 21.11.2020 02:30

History, 21.11.2020 02:30


Social Studies, 21.11.2020 02:30


Mathematics, 21.11.2020 02:30