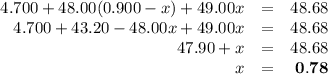Chemistry, 12.09.2019 21:30 SuperWoman9172
Ahypothetical element has an atomic weight of 48.68 amu. it consists of three isotopes having masses of 47.00 amu, 48.00 amu, and 49.00 amu. the lightest-weight isotope has a natural abundance of 10.0%. what is the percent abundance of the heaviest isotope?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
Ahypothetical element has an atomic weight of 48.68 amu. it consists of three isotopes having masses...
Questions


Health, 20.10.2020 02:01

Mathematics, 20.10.2020 02:01



Mathematics, 20.10.2020 02:01

Mathematics, 20.10.2020 02:01

Mathematics, 20.10.2020 02:01


Mathematics, 20.10.2020 02:01


Mathematics, 20.10.2020 02:01

Mathematics, 20.10.2020 02:01


Geography, 20.10.2020 02:01

French, 20.10.2020 02:01



History, 20.10.2020 02:01