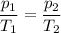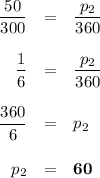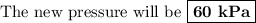Chemistry, 12.09.2019 21:30 emmarieasimon
if gas in a sealed container has a pressure of 50 kpa at 300 k, what will the pressure be if the temperature rises to 360 k?
60 kpa
161 kpa
41 kpa
16 kpa

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1
You know the right answer?
if gas in a sealed container has a pressure of 50 kpa at 300 k, what will the pressure be if the tem...
Questions


Geography, 30.09.2020 02:01

Health, 30.09.2020 02:01





French, 30.09.2020 02:01




Mathematics, 30.09.2020 02:01

Mathematics, 30.09.2020 02:01

Chemistry, 30.09.2020 02:01



Mathematics, 30.09.2020 02:01