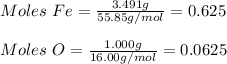Chemistry, 12.09.2019 21:30 dacanul100
Two iron oxide samples are given to you where one is red and the other is black. you perform a chemical analysis and you find that the red sample has a fe/o mass ratio of 2.327 and the black has a fe/o mass ratio of 3.491. you suspect the red sample is simple rust or fe2o3. what is the chemical formula for the black sample?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

You know the right answer?
Two iron oxide samples are given to you where one is red and the other is black. you perform a chemi...
Questions



Engineering, 05.11.2019 04:31


Mathematics, 05.11.2019 04:31

Mathematics, 05.11.2019 04:31


History, 05.11.2019 04:31


Chemistry, 05.11.2019 04:31



Mathematics, 05.11.2019 04:31

Mathematics, 05.11.2019 04:31

Mathematics, 05.11.2019 04:31

History, 05.11.2019 04:31

English, 05.11.2019 04:31

Mathematics, 05.11.2019 04:31

Chemistry, 05.11.2019 04:31