
Chemistry, 12.09.2019 21:30 Benjamincompton07
Atechnician tares a 100.0 ml volumetric flask at 150.00 g. after adding sodium chloride to the flask it then weighs 158.84 g. assuming an error of 0.2 ml in the volumetric volume and 0.005 g in the weight, calculate the molar concentration of sodium chloride and its associated standard deviation.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
You know the right answer?
Atechnician tares a 100.0 ml volumetric flask at 150.00 g. after adding sodium chloride to the flask...
Questions

Chemistry, 19.12.2020 09:10

Physics, 19.12.2020 09:10

Mathematics, 19.12.2020 09:10

Mathematics, 19.12.2020 09:10

Mathematics, 19.12.2020 09:10


Mathematics, 19.12.2020 09:10

Arts, 19.12.2020 09:10






Mathematics, 19.12.2020 09:20

Mathematics, 19.12.2020 09:20




History, 19.12.2020 09:20



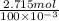 mol/liter
mol/liter mol/liter
mol/liter 

