Find the theoretical oxygen demand for the
followingsolutions?
a. 200mg/l of acetic aci...

Chemistry, 13.09.2019 17:30 davistakeisha95
Find the theoretical oxygen demand for the
followingsolutions?
a. 200mg/l of acetic acid, ch3cooh.
b. 30mg/l of ethanol, c2h5oh.
c. 50mg/l of sucrose c2h12o6.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2
You know the right answer?
Questions


Geography, 13.10.2019 01:30

World Languages, 13.10.2019 01:30

English, 13.10.2019 01:30

History, 13.10.2019 01:30

Biology, 13.10.2019 01:30

Mathematics, 13.10.2019 01:30

Mathematics, 13.10.2019 01:30

Mathematics, 13.10.2019 01:30




Mathematics, 13.10.2019 01:30

Physics, 13.10.2019 01:30

History, 13.10.2019 01:30


Mathematics, 13.10.2019 01:30

Mathematics, 13.10.2019 01:30

Mathematics, 13.10.2019 01:30

![[CH3COOH]](/tpl/images/0229/9730/1e5f9.png) = 200 mg/L
= 200 mg/L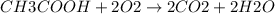
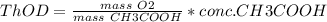
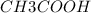 = 60 g
= 60 g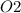 = 2(32) = 64 g
= 2(32) = 64 g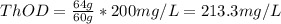
![[C2H5OH]](/tpl/images/0229/9730/4a8a7.png) = 30 mg/L
= 30 mg/L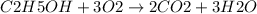

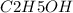 = 46 g
= 46 g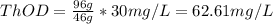
![[C12H22O11]](/tpl/images/0229/9730/ff3eb.png) = 50 mg/L
= 50 mg/L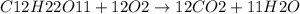

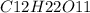 = 342 g
= 342 g



