
Chemistry, 13.09.2019 17:30 michaelbromley9759
Hydrogen reacts with sodium to produce solid sodium hydride. a reaction mixture contains 6.75 g na and 3.03 g hydrogen. which reactant is limiting?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2
You know the right answer?
Hydrogen reacts with sodium to produce solid sodium hydride. a reaction mixture contains 6.75 g na a...
Questions






Mathematics, 05.12.2019 19:31














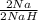 = 0.294mol NaH
= 0.294mol NaH


