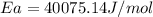Chemistry, 13.09.2019 20:30 solphiafischer
The rate of reaction at 550 k is ten times faster than the rate of reaction at 440 k. find the activation energy from the collision theory. a) 40075.14 j/mol b) 50078.5j/mol c) 44574.5 j/mol d) 43475.5 j/mol

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1


Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
You know the right answer?
The rate of reaction at 550 k is ten times faster than the rate of reaction at 440 k. find the activ...
Questions



Mathematics, 07.04.2020 06:11

Biology, 07.04.2020 06:11

Mathematics, 07.04.2020 06:11

Biology, 07.04.2020 06:11

Mathematics, 07.04.2020 06:11



Mathematics, 07.04.2020 06:11




Social Studies, 07.04.2020 06:11




Mathematics, 07.04.2020 06:11

Law, 07.04.2020 06:11

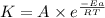
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0230/1925/6d953.png)
 = rate constant at
= rate constant at  = k
= k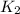 = rate constant at
= rate constant at 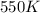 = 10 k
= 10 k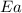 = activation energy for the reaction = ?
= activation energy for the reaction = ?
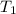 = initial temperature = 440 K
= initial temperature = 440 K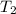 = final temperature = 550 K
= final temperature = 550 K![\log (\frac{10k}{k})=\frac{Ea}{2.303\times 8.314J/mole.K}[\frac{1}{440K}-\frac{1}{550K}]](/tpl/images/0230/1925/ff915.png)