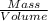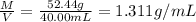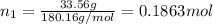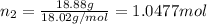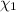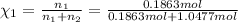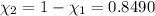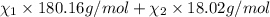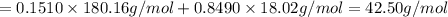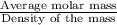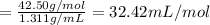Chemistry, 13.09.2019 21:30 sindy35111
33.56 g of fructose (c6h,206) and 18.88 g of water are mixed to obtain a 40.00 ml solution a. what is this solution's density? b. what is the mole fraction of fructose in this solution? c. what is the solution's average molar mass? d. what is the specific molar volume of the solution?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2


Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1
You know the right answer?
33.56 g of fructose (c6h,206) and 18.88 g of water are mixed to obtain a 40.00 ml solution a. what i...
Questions


Business, 31.07.2019 06:30





History, 31.07.2019 06:30

History, 31.07.2019 06:30

Business, 31.07.2019 06:30


Biology, 31.07.2019 06:30


Spanish, 31.07.2019 06:30

Spanish, 31.07.2019 06:30

Spanish, 31.07.2019 06:30


Spanish, 31.07.2019 06:30