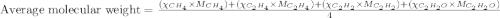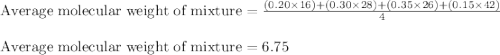Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
Aliquid mixture composed of 20% ch4, 30% c2h4, 35% c2h2, and 15% c2h20. what is the average molecula...
Questions


Mathematics, 19.08.2019 12:10



English, 19.08.2019 12:10

Mathematics, 19.08.2019 12:10




Mathematics, 19.08.2019 12:10

Social Studies, 19.08.2019 12:10





Mathematics, 19.08.2019 12:10


Biology, 19.08.2019 12:10


 = 20 %
= 20 %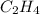 = 30 %
= 30 %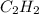 = 35 %
= 35 % = 15 %
= 15 %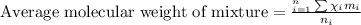
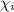 = mole fractions of i-th species
= mole fractions of i-th species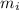 = molar masses of i-th species
= molar masses of i-th species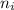 = number of observations
= number of observations