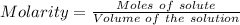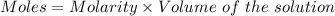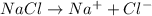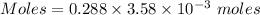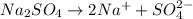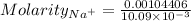Chemistry, 13.09.2019 22:30 jazz589729
Calculate the molarity of sodium ion in a solution made
bymixing 3.58 ml of 0.288 m sodium chloride with 500 ml of 6.51
times1/1000 m sodium sulfate ( assume volumes are additive ).

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2
You know the right answer?
Calculate the molarity of sodium ion in a solution made
bymixing 3.58 ml of 0.288 m sodium chl...
bymixing 3.58 ml of 0.288 m sodium chl...
Questions

Mathematics, 23.04.2020 21:41


Mathematics, 23.04.2020 21:41

Mathematics, 23.04.2020 21:41

Mathematics, 23.04.2020 21:41

Mathematics, 23.04.2020 21:41

History, 23.04.2020 21:41

Biology, 23.04.2020 21:41

Computers and Technology, 23.04.2020 21:41

History, 23.04.2020 21:41

Biology, 23.04.2020 21:41


Mathematics, 23.04.2020 21:41



English, 23.04.2020 21:41

Mathematics, 23.04.2020 21:41



Mathematics, 23.04.2020 21:41