
Chemistry, 13.09.2019 22:30 sosick90501
Question: dimethylhydrazine, the
fuel used in the apollo lunar descentmodule, has a molar mass of
60.10 g/mol. it is made up of carbon, hydrogen, and nitrogen atoms.
the combustion of 2.859g of the fuelin excess oxygen yields 4.190g
of carbon dioxideand 3.428g ofwater. what are the simplest and
molecular formulas fordimethylhydrazine?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2


Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
You know the right answer?
Question: dimethylhydrazine, the
fuel used in the apollo lunar descentmodule, has a molar mass...
fuel used in the apollo lunar descentmodule, has a molar mass...
Questions

English, 23.09.2020 14:01


Mathematics, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01

Arts, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01


Mathematics, 23.09.2020 14:01

Spanish, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01


Mathematics, 23.09.2020 14:01


Health, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01


Mathematics, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01

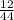 x 4.19g = 1.14g
x 4.19g = 1.14g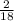 x 3.428g = 0.38g
x 3.428g = 0.38g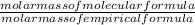 =
= 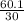 = 2
= 2

