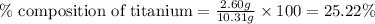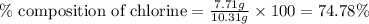Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1
You know the right answer?
A2.60 g sample of titanium metal chemically combines
withchlorine gas to form 10.31g of a tita...
withchlorine gas to form 10.31g of a tita...
Questions

History, 22.04.2020 03:36






Mathematics, 22.04.2020 03:36






Computers and Technology, 22.04.2020 03:37




Social Studies, 22.04.2020 03:37



History, 22.04.2020 03:37

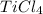
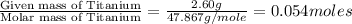
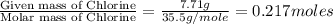
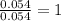
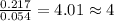
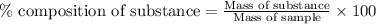 .......(1)
.......(1)