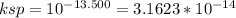Calculate the number of mg of mn2+ left
unprecipitated in 100 ml of a 0.1000m solution of mnso...

Chemistry, 13.09.2019 22:30 praptibaral70
Calculate the number of mg of mn2+ left
unprecipitated in 100 ml of a 0.1000m solution of mnso4
to whichenough na2s has been added to makethe final
sulfide ion (s2-)concentration equal to 0.0900 m. assume
no change in volume due tothe addition of na2s.
thepksp of mns is 13.500.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
Questions

Mathematics, 17.10.2020 03:01



Arts, 17.10.2020 03:01

Mathematics, 17.10.2020 03:01

Mathematics, 17.10.2020 03:01


English, 17.10.2020 03:01

Mathematics, 17.10.2020 03:01


Mathematics, 17.10.2020 03:01

English, 17.10.2020 03:01


History, 17.10.2020 03:01

Advanced Placement (AP), 17.10.2020 03:01



Mathematics, 17.10.2020 03:01

English, 17.10.2020 03:01

Mathematics, 17.10.2020 03:01