
To find the formula of a compound composed of iron and carbon monoxide, fex(co)y, the compound is burned in pure oxygen, an reaction that proceeds according to the following unbalanced equation.
fex(co)y + o2 --> fe2o3 + co2
if you burn 1.959 g. of fex(co)y and obtain 0.799 g. of fe2o3 and 2.200 g. of co2, what is the empirical formula of fex(co)y?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1
You know the right answer?
To find the formula of a compound composed of iron and carbon monoxide, fex(co)y, the compound is bu...
Questions







Mathematics, 13.04.2020 23:20



Mathematics, 13.04.2020 23:20



Mathematics, 13.04.2020 23:20


Computers and Technology, 13.04.2020 23:21



English, 13.04.2020 23:21


Mathematics, 13.04.2020 23:21

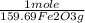 = 5,00×10⁻³ Fe₂O₃ moles
= 5,00×10⁻³ Fe₂O₃ moles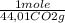 = 5,00×10⁻²CO₂ moles
= 5,00×10⁻²CO₂ moles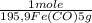 = 1,00×10⁻² Fe(CO)₅ moles
= 1,00×10⁻² Fe(CO)₅ moles

