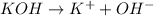Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

You know the right answer?
How do you calculate the ph of a 1.6 m koh solution?...
Questions

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

English, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Arts, 18.09.2020 04:01

Biology, 18.09.2020 04:01

Social Studies, 18.09.2020 04:01

English, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01

Mathematics, 18.09.2020 04:01