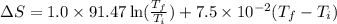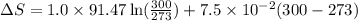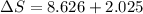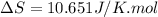in the range 240k to 330k is given

Chemistry, 13.09.2019 23:10 mariaaalopezz
The heat capacity of chloroform (trichloromethane, chcl3)
in the range 240k to 330k is given
bycpm/(jk-1mol-1) = 91.47
+7.5x10-2(t/k). in a particular experiment,
1.0molchcl3 is heated from 273k to 300k. calculate the
changein molar entropy of the sample.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1
You know the right answer?
The heat capacity of chloroform (trichloromethane, chcl3)
in the range 240k to 330k is given
in the range 240k to 330k is given
Questions

Mathematics, 28.09.2020 01:01



English, 28.09.2020 01:01

Mathematics, 28.09.2020 01:01


Mathematics, 28.09.2020 01:01


Social Studies, 28.09.2020 01:01

History, 28.09.2020 01:01

Mathematics, 28.09.2020 01:01

Mathematics, 28.09.2020 01:01

Computers and Technology, 28.09.2020 01:01


Biology, 28.09.2020 01:01



Mathematics, 28.09.2020 01:01


Mathematics, 28.09.2020 01:01

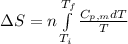
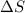 = change in molar entropy
= change in molar entropy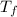 = final temperature = 300 K
= final temperature = 300 K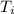 = initial temperature = 273 K
= initial temperature = 273 K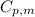 = heat capacity of chloroform =
= heat capacity of chloroform = 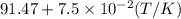
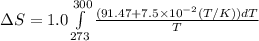
![\Delta S=1.0\times [91.47\ln T+7.5\times 10^{-2}T]^{300}_{273}](/tpl/images/0230/3981/c380e.png)