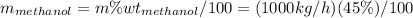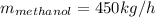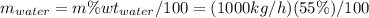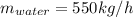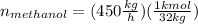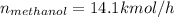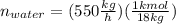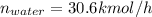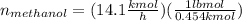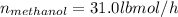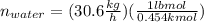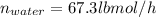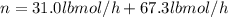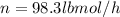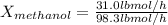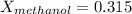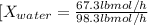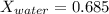Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1
You know the right answer?
Conversion of mass to moles a continuous feed to a separation unit is 1,000 kg/h of 45 wt% methanol...
Questions



Mathematics, 29.12.2019 11:31

Mathematics, 29.12.2019 11:31

Mathematics, 29.12.2019 11:31

Social Studies, 29.12.2019 11:31


Mathematics, 29.12.2019 11:31



Biology, 29.12.2019 12:31


History, 29.12.2019 12:31


History, 29.12.2019 12:31

Geography, 29.12.2019 12:31

Geography, 29.12.2019 12:31



English, 29.12.2019 12:31