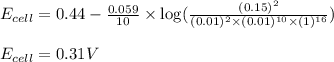Chemistry, 13.09.2019 23:20 michelle230
Calculate the cell potential for a cell operating with the following reaction at 25 degrees celsius, in which [mno4^1-] = .01m, [br^1-] = .01m, [mn^2+] = .15m, and [h^1+] = 1m. the reaction is 2 mno4^1-(aq) + 10 br^1-(aq) + 16 h^1+(aq) --> 2 mn^2+(aq) + 5 br2(l) + 8 h2o(l)

Answers: 1
Another question on Chemistry

Chemistry, 20.06.2019 18:02
Chemistry answer as much as you can if you can't answer more then 2 don't answer
Answers: 2


Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
You know the right answer?
Calculate the cell potential for a cell operating with the following reaction at 25 degrees celsius,...
Questions





Mathematics, 01.07.2019 01:40

Mathematics, 01.07.2019 01:40



Mathematics, 01.07.2019 01:40



World Languages, 01.07.2019 01:40


History, 01.07.2019 01:40

Chemistry, 01.07.2019 01:40



History, 01.07.2019 01:40

Mathematics, 01.07.2019 01:40

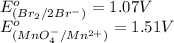
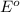 potential will always get reduced and will undergo reduction reaction. Here,
potential will always get reduced and will undergo reduction reaction. Here, 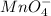 will undergo reduction reaction will get reduced. And, bromine will get oxidized.
will undergo reduction reaction will get reduced. And, bromine will get oxidized.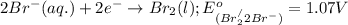 ( × 5)
( × 5)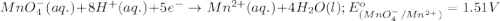 ( × 2)
( × 2)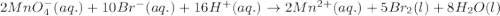
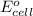 of the reaction, we use the equation:
of the reaction, we use the equation: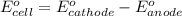
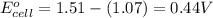
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Mn^{2+}]^2}{[MnO_4^{-}]^2\times [Br^-]^{10}\times [H^+]^{16}}](/tpl/images/0230/4138/86671.png)
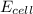 = electrode potential of the cell = ?V
= electrode potential of the cell = ?V![[H^{+}]=1M](/tpl/images/0230/4138/c7b74.png)
![[Mn^{2+}]=0.15M](/tpl/images/0230/4138/f060a.png)
![[MnO_4^{-}]=0.01M](/tpl/images/0230/4138/b97e7.png)
![[Br^{-}]=0.01M](/tpl/images/0230/4138/bb1d7.png)