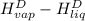Chemistry, 13.09.2019 23:20 SkyeShadow525
The halpy of vaporization of h2o at 1 atm and 100 c is 2259 kj/kg. the heat capacity of liquid water is 4.19 kj/kg. c, and the heat capacity of water vapor is 1.9 kj/kg-c. h20 at 10 bar boils at 179.9 c. what is the enthalpy of vaporization of h20 at 10 bar? you can neglect the effect of pressure. e 2076 kj/kg e 1924 kj/kg e 2259 kj/kg 2442 kj/kg 2594 kj/kg none of the above

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 23.06.2019 05:00
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2
You know the right answer?
The halpy of vaporization of h2o at 1 atm and 100 c is 2259 kj/kg. the heat capacity of liquid water...
Questions





Health, 20.04.2021 18:10



Mathematics, 20.04.2021 18:10

Social Studies, 20.04.2021 18:10




Mathematics, 20.04.2021 18:10

English, 20.04.2021 18:10





Mathematics, 20.04.2021 18:10

Health, 20.04.2021 18:10

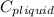 = 4.19
= 4.19 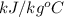
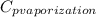 = 1.9
= 1.9 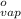 ) at 1 atm and
) at 1 atm and 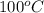 is 2259 kJ/kg
is 2259 kJ/kg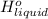 = 0
= 0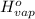 =
= 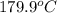 and effect of pressure is not considered. Hence, enthalpy of liquid water at 10 bar and
and effect of pressure is not considered. Hence, enthalpy of liquid water at 10 bar and 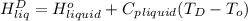
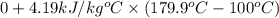
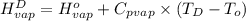
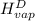 =
= 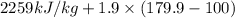
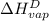 =
=