Prob.: consider the combustion of butane (c4h10):
2c4h10(g) + 13o2(g) ==> 8co2(g) + 10 h...

Chemistry, 14.09.2019 00:30 zabomoxx5ll
Prob.: consider the combustion of butane (c4h10):
2c4h10(g) + 13o2(g) ==> 8co2(g) + 10 h2o(l)
in a particular reaction, 5.0 moles of c4h10 are reacted
withan excess of o2. calculate the number of moles of co2
formed.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

You know the right answer?
Questions

English, 28.04.2021 14:00

History, 28.04.2021 14:00

Physics, 28.04.2021 14:00





English, 28.04.2021 14:00



Mathematics, 28.04.2021 14:00



Computers and Technology, 28.04.2021 14:00


Mathematics, 28.04.2021 14:00

Mathematics, 28.04.2021 14:00

Biology, 28.04.2021 14:00


Biology, 28.04.2021 14:00

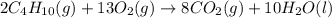
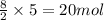 of carbon dioxide.
of carbon dioxide.

