
Chemistry, 25.12.2019 20:31 tommylopez22713
Consider the following reversible, exothermic chemical reaction: 2c2h2(g) + 5o2(g) ↔4co2(g) + 2h2o(g)
all but one of the conditions will cause equilibrium to shift right and produce more of the products. which change will cause the reaction to shift to the left?
a) adding oxygen
b) adding co2
c) increasing the pressure
d) decreasing the temperature

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3


Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
You know the right answer?
Consider the following reversible, exothermic chemical reaction: 2c2h2(g) + 5o2(g) ↔4co2(g) + 2h2o(...
Questions


History, 15.02.2021 23:00

Chemistry, 15.02.2021 23:00



Mathematics, 15.02.2021 23:00


Medicine, 15.02.2021 23:00





Mathematics, 15.02.2021 23:00



Spanish, 15.02.2021 23:00

Spanish, 15.02.2021 23:00

Mathematics, 15.02.2021 23:00


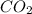
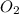 is increased on reactant side then the equilibrium will shift in the direction where decrease of concentration of
is increased on reactant side then the equilibrium will shift in the direction where decrease of concentration of 

