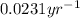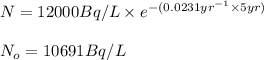Chemistry, 14.09.2019 00:30 natalia9573
Immediately after the chernobyl nuclear accident, the concentratiorn of 137cs (cesium 137) in cow's milk was 12,000 bq/l (a becquerel is a measure of radioactivity; one becquerel equals one radioactive disintegration per second). assume that the only reaction by which the 137cs was lost from the soil was through radioactive decay. also assume that the concentration in cows milk is directly proportional to the concentration in the soil. calculate the concentration of 137cs in cow's milk (from feeding on grass in the soil) 5 years after the accident given a half-life for 137cs of 30 years.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
Immediately after the chernobyl nuclear accident, the concentratiorn of 137cs (cesium 137) in cow's...
Questions

Advanced Placement (AP), 16.07.2019 05:00

Mathematics, 16.07.2019 05:00

Mathematics, 16.07.2019 05:00

English, 16.07.2019 05:00

Mathematics, 16.07.2019 05:00


Social Studies, 16.07.2019 05:00



English, 16.07.2019 05:00

English, 16.07.2019 05:00

Mathematics, 16.07.2019 05:00

Mathematics, 16.07.2019 05:00

Mathematics, 16.07.2019 05:00



Mathematics, 16.07.2019 05:00

History, 16.07.2019 05:00


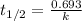
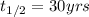
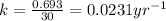
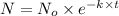
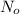 = initial concentration of Cow's milk = 12000 Bq/L
= initial concentration of Cow's milk = 12000 Bq/L