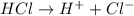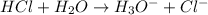Chemistry, 14.09.2019 06:30 theresamarieuehling2
Define the following: bronsted-lowry acid - lewis acid- strong acid - (5 points) problem 6: consider the following acid base reaction hci + h20 → h30+ + cl- a) is this a strong acid? b) clearly label the acid, base, conjugate acid and conjugate base. (5 points)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:20
Concerning the 10.0 ml of 0.50 m nacl to 100 ml of solution: when a solution is diluted, does it change the number of moles dissolved?
Answers: 3

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2


Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1
You know the right answer?
Define the following: bronsted-lowry acid - lewis acid- strong acid - (5 points) problem 6: consid...
Questions


Arts, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10




Mathematics, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10

History, 18.03.2021 01:10


Mathematics, 18.03.2021 01:10






Social Studies, 18.03.2021 01:10



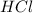 is a strong acid.
is a strong acid. 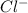 , base =
, base = 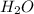 , conjugate acid =
, conjugate acid = 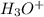
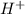 ions.
ions.