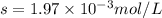Calcium sulfate is only sparingly soluble. caso4(s) ⇌ ca2+(aq) + so42-(aq) for this type of dissolution reaction the equilibrium constant, also known as the solubility product, is denoted ks. in the reaction above, ks = 3.9 x 10-6. when an excess of the solid is dissolved in water what is the maximum concentration of ca2+(aq) in mol l-1?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2
You know the right answer?
Calcium sulfate is only sparingly soluble. caso4(s) ⇌ ca2+(aq) + so42-(aq) for this type of dissolut...
Questions


Mathematics, 19.09.2019 04:00

English, 19.09.2019 04:00

History, 19.09.2019 04:00


Mathematics, 19.09.2019 04:00



Mathematics, 19.09.2019 04:00



History, 19.09.2019 04:00


Mathematics, 19.09.2019 04:00



Mathematics, 19.09.2019 04:00

Mathematics, 19.09.2019 04:00


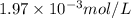
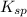
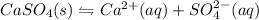
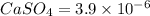
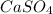 gives 1 mole of
gives 1 mole of 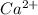 and 1 mole of
and 1 mole of 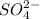 .
.![K_{sp}=[Ca^{2+}][SO_4^{2-}]](/tpl/images/0231/0233/958f2.png)
![3.9\times 10^{-6}=[s][s]](/tpl/images/0231/0233/47679.png)