
Chemistry, 14.09.2019 07:10 isaaccott013
What is the mass, in pounds, of 389 ml of a gas that has a density of 1.29 g/l?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
What is the mass, in pounds, of 389 ml of a gas that has a density of 1.29 g/l?...
Questions

History, 10.02.2022 08:20

Mathematics, 10.02.2022 08:20

Social Studies, 10.02.2022 08:20


Mathematics, 10.02.2022 08:20

Mathematics, 10.02.2022 08:20

Mathematics, 10.02.2022 08:20



Mathematics, 10.02.2022 08:20


Mathematics, 10.02.2022 08:20

Social Studies, 10.02.2022 08:20


Social Studies, 10.02.2022 08:20



Chemistry, 10.02.2022 08:30

English, 10.02.2022 08:30

Computers and Technology, 10.02.2022 08:30

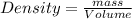
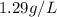
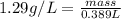
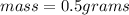
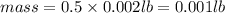 (1g =0.002 lb)
(1g =0.002 lb)

