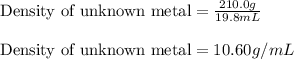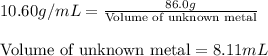Chemistry, 14.09.2019 07:30 gmaxgaming88
Apiece of an unknown metal has a volume of 19.8 ml and a mass of 210.0 grams. the density of the metal is g/ml a piece of the same metal with a mass of 86.0 grams would have a volume of ml.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3


Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

You know the right answer?
Apiece of an unknown metal has a volume of 19.8 ml and a mass of 210.0 grams. the density of the met...
Questions



Mathematics, 02.02.2020 19:54






Physics, 02.02.2020 19:54





History, 02.02.2020 19:54

Business, 02.02.2020 19:54

Mathematics, 02.02.2020 19:54


History, 02.02.2020 19:54

History, 02.02.2020 19:54

Mathematics, 02.02.2020 19:54

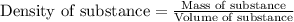 ......(1)
......(1)