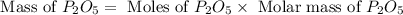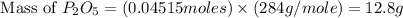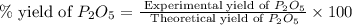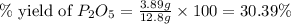Chemistry, 14.09.2019 07:30 holycow7868
Determine the percent yield of the following reaction when 2.80 g of p reacts with excess oxygen. the actual yield of this reaction is determined to by 3.89 g of p2o5.
4 p + 5 o2 > 2 p2o5

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:00
How has the scientific community addressed the safety of chemicals? a. chemicals are repeatedly tested, even those that have existed for a long time. b. existing chemicals are tested if they have never been tested before. c. chemicals are tested if they are suspected to have caused a problem. d. only new chemicals are tested.
Answers: 2


Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 23.06.2019 08:30
Kelly has come up with an explanation for why her sister is sometimes in a good mood and other times in a bad mood. she speculates that it is based on the hours of sleep her sister got the previous night. this explanation for her sister's behaviors is an example of a(n)
Answers: 3
You know the right answer?
Determine the percent yield of the following reaction when 2.80 g of p reacts with excess oxygen. th...
Questions

English, 19.04.2021 19:00

Mathematics, 19.04.2021 19:00

Social Studies, 19.04.2021 19:00

History, 19.04.2021 19:00




Mathematics, 19.04.2021 19:00


Biology, 19.04.2021 19:00


Biology, 19.04.2021 19:00


Mathematics, 19.04.2021 19:00

Biology, 19.04.2021 19:00



Mathematics, 19.04.2021 19:00

History, 19.04.2021 19:00

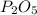 is, 30.39 %
is, 30.39 %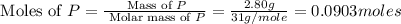
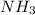
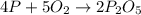
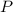 react to give 2 mole of
react to give 2 mole of 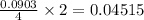 moles of
moles of