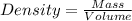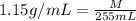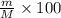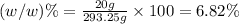Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1

You know the right answer?
Asolution is prepared by dissolving 20 g naoh (fw = 400 g/mol) to 255 ml of solution. if the density...
Questions

Mathematics, 09.10.2021 15:40


Mathematics, 09.10.2021 15:40

English, 09.10.2021 15:40



History, 09.10.2021 15:40

Mathematics, 09.10.2021 15:40


History, 09.10.2021 15:50

English, 09.10.2021 15:50

Mathematics, 09.10.2021 15:50

Mathematics, 09.10.2021 15:50




Mathematics, 09.10.2021 15:50