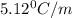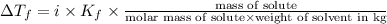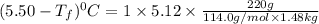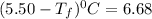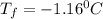Chemistry, 14.09.2019 08:30 lineaeriksen
Calculate the freezing point of a solution made from 220g of octane (c hua), molar mass = 114,0 gmol dissolved in 1480 g of benzene. benzene freezes at 5.50"c and its kvalue is 5.12c/m. -1.16°c 0.98°c 666"c 12 2°c 5.49°c 10 12 am a a 2019 backspace yuo pill но кl

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3


Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2

You know the right answer?
Calculate the freezing point of a solution made from 220g of octane (c hua), molar mass = 114,0 gmol...
Questions





History, 13.09.2019 18:30

Biology, 13.09.2019 18:30





Mathematics, 13.09.2019 18:30

English, 13.09.2019 18:30

Mathematics, 13.09.2019 18:30

Mathematics, 13.09.2019 18:30


Social Studies, 13.09.2019 18:30



Mathematics, 13.09.2019 18:30

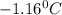
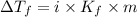
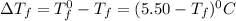 = Depression in freezing point
= Depression in freezing point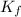 = freezing point constant =
= freezing point constant =