
Chemistry, 14.09.2019 08:30 brianamitzel1013
500 ml of a solution contains 1000 mg of cacl2. molecular weight of cacl2 is 110 g/mol. specific gravity of the solution is 0. cacl2 = ca++ + 2cl-
a) express the concentration of the solution in % w/v
b) express the concentration in ratio strength
c) express the concentration in molarity (m)
d) express the concentration in molality (m)
e) how many equivalents of calcium chloride would be in 1.5 l of the solution?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3


Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1
You know the right answer?
500 ml of a solution contains 1000 mg of cacl2. molecular weight of cacl2 is 110 g/mol. specific gra...
Questions


Mathematics, 19.12.2019 09:31

Biology, 19.12.2019 09:31

Mathematics, 19.12.2019 09:31

Chemistry, 19.12.2019 09:31

Biology, 19.12.2019 09:31

Mathematics, 19.12.2019 09:31

Mathematics, 19.12.2019 09:31

Mathematics, 19.12.2019 09:31

Biology, 19.12.2019 09:31

Mathematics, 19.12.2019 09:31


Mathematics, 19.12.2019 09:31

Mathematics, 19.12.2019 09:31


Mathematics, 19.12.2019 09:31

Mathematics, 19.12.2019 09:31

Mathematics, 19.12.2019 09:31

English, 19.12.2019 09:31

Mathematics, 19.12.2019 09:31

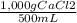 ×100 = 0,2%w/v
×100 = 0,2%w/v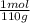 = 9,09×10⁻³ moles of CaCl₂
= 9,09×10⁻³ moles of CaCl₂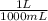 = 0,500 L of solution
= 0,500 L of solution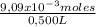 = 0,0182 M
= 0,0182 M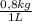 = 0,625 kg of solution
= 0,625 kg of solution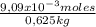 = 0,0145 m
= 0,0145 m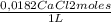 ×
× 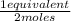 = 0,0137 equivalents
= 0,0137 equivalents

