
Chemistry, 14.09.2019 08:30 xxaurorabluexx
Look at sample problem 17.10 in the 8th edition silberberg book.
the research and development unit of a chemical company is studying the reaction of methane and h2s, two components of natural gas:
ch4 (g) + 2 h2s (g) ⇋ cs2 (g) + 4 h2 (g)
in one experiment, 1.0 mol of ch4, 1.0 mol of cs2, 2.0 mol of h2s, and 2.0 mol of h2 are mixed in a 400. ml vessel at 960°c. at this temperature, kc = 225.
in which direction will the reaction proceed to reach equilibrium? enter right, left, or at equilibrium,
if the concentration of methane at equilibrium is 2.0 m, what is the equilibrium concentration of h2? enter a number to 1 decimal places.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
Look at sample problem 17.10 in the 8th edition silberberg book.
the research and development...
the research and development...
Questions




History, 21.09.2019 22:50

Chemistry, 21.09.2019 22:50


Biology, 21.09.2019 22:50

History, 21.09.2019 22:50

Mathematics, 21.09.2019 22:50






Social Studies, 21.09.2019 22:50

Biology, 21.09.2019 22:50

Mathematics, 21.09.2019 22:50



English, 21.09.2019 22:50

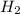 is 7.0 M
is 7.0 M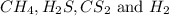 .
.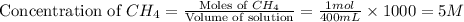
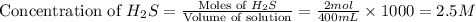
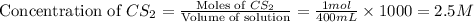
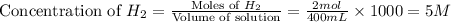
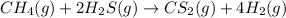
![Q_c=\frac{[CS_2][H_2]^4}{[CH_4][H_2S]^2}](/tpl/images/0231/1253/b2aa9.png)
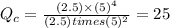
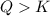 that means product > reactant. So, the reaction is reactant favored.
that means product > reactant. So, the reaction is reactant favored.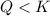 that means reactant > product. So, the reaction is product favored.
that means reactant > product. So, the reaction is product favored.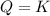 that means product = reactant. So, the reaction is in equilibrium.
that means product = reactant. So, the reaction is in equilibrium.
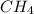 at equilibrium = 2.0 M
at equilibrium = 2.0 M

