
Chemistry, 14.09.2019 09:30 Fangflora3
Question 10 0 / 3.5 points many high temperature studies have been carried out on the equilibrium of the reaction: 2so2(g) + o2(g) = 2 so3(g) in one study the reaction vessel initially contained (5.000x10^-3) m so2, (2.50x10^-3) mo2, and no so3. if it was determined that at equilibrium the so2 concentration was (2.8x10^-3) m, determine kc at this temperature for the reaction as written. • answers must be written in scientific notation • write your answer using one decimal place (two significant figures), even if this is not the correct number of significant figures (e. g., 3.4e-6 or 3.4 x 10-6). • do not use spaces. • do not include units.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1


Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
Question 10 0 / 3.5 points many high temperature studies have been carried out on the equilibrium of...
Questions

Business, 04.11.2019 22:31














Computers and Technology, 04.11.2019 22:31




Computers and Technology, 04.11.2019 22:31

Chemistry, 04.11.2019 22:31


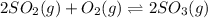
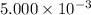
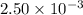 0
0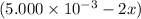
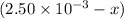 (2x)
(2x) 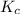 for the given reaction follows:
for the given reaction follows:![K_c=\frac{[SO_3]^2}{[SO_2]^2[O_2]}](/tpl/images/0231/2030/1f129.png)
![K_c=\frac{[2x]^2}{[5.000\times 10^{-3}-2x]^2[2.50\times 10^{-3}-x]}](/tpl/images/0231/2030/4b90a.png)
![[SO_2]_{eqm}=2.80\times 10^{-3}](/tpl/images/0231/2030/39bea.png)


![K_c=\frac{[2\times 1.1\times 10^{-3}]^2}{[5.000\times 10^{-3}-2\times 1.1\times 10^{-3}]^2[2.50\times 10^{-3}-1.1\times 10^{-3}]}](/tpl/images/0231/2030/4996b.png)



