
Chemistry, 14.09.2019 09:30 babygurl27732
If 1.0 m hcl is added to an equal volume of 0.2 m naoh, what are the new concentrations of oh and h30+ ? select one: [h30*] = 0.8 m, [oh]= 1.3 x 10-14 m h30*] = 0.5 m, [oh]= 0.1 mi h30*] = 0.4 m, [oh]= 2.5 x 1014 m h30*] 1.0 m, [0h]= 0.2 m h30*] = 0.5 m, [oh]= 2.0 x 1014 m

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1


Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1
You know the right answer?
If 1.0 m hcl is added to an equal volume of 0.2 m naoh, what are the new concentrations of oh and h3...
Questions

Mathematics, 18.09.2021 02:00

English, 18.09.2021 02:00


Health, 18.09.2021 02:00


Mathematics, 18.09.2021 02:00




History, 18.09.2021 02:00

Mathematics, 18.09.2021 02:00


Mathematics, 18.09.2021 02:00

Mathematics, 18.09.2021 02:00


Mathematics, 18.09.2021 02:00

Mathematics, 18.09.2021 02:00



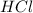 is a strong acid and
is a strong acid and 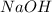 is a strong base. The resulting reaction is a neutralization reaction forming
is a strong base. The resulting reaction is a neutralization reaction forming 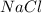 and
and 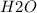

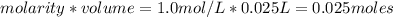
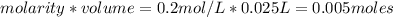
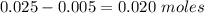
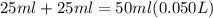
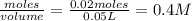
![[H3O+][OH-] = 10^{-14}\\ \\[OH-] = \frac{10^{-14} }{[H3O+]} = \frac{10^{-14} }{0.4} =2.5*10^{-14}](/tpl/images/0231/2255/824f2.png)


