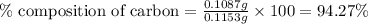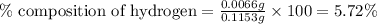Chemistry, 14.09.2019 09:30 bagofmud8339
A0.1153-gram sample of a pure hydrocarbon was burned in a c-h combustion train to produce 0.3986 gram of co2and 0.0578 gram of h2o. determine the masses of c and h in the sample and the percentages of these elements in this hydrocarbon.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1
You know the right answer?
A0.1153-gram sample of a pure hydrocarbon was burned in a c-h combustion train to produce 0.3986 gra...
Questions


Mathematics, 08.04.2021 15:50


History, 08.04.2021 15:50

Mathematics, 08.04.2021 15:50


Mathematics, 08.04.2021 15:50

Geography, 08.04.2021 15:50


History, 08.04.2021 15:50


History, 08.04.2021 15:50



English, 08.04.2021 15:50



SAT, 08.04.2021 15:50

Geography, 08.04.2021 15:50

Mathematics, 08.04.2021 15:50

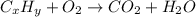
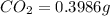
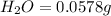
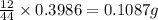 of carbon will be contained.
of carbon will be contained.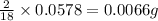 of hydrogen will be contained.
of hydrogen will be contained.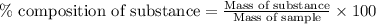 ......(1)
......(1)