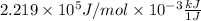Chemistry, 14.09.2019 09:30 victoriadorvilu
The vapor pressure of substance x is 100. mm hg at 1080.°c. the vapor pressure of substance x increases to 600. mm hg at 1220.°c. determine the molar heat of vaporization of substance x using the derived form of the clausius-clapeyron equation given below. (include the sign of the value in your answer.) kj/mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
The vapor pressure of substance x is 100. mm hg at 1080.°c. the vapor pressure of substance x increa...
Questions


Biology, 25.12.2020 03:50


Mathematics, 25.12.2020 04:00


Mathematics, 25.12.2020 04:20

English, 25.12.2020 04:20

English, 25.12.2020 04:20

History, 25.12.2020 04:20

Biology, 25.12.2020 04:20

Computers and Technology, 25.12.2020 04:20

Business, 25.12.2020 04:20

Business, 25.12.2020 04:20

Biology, 25.12.2020 04:20

Mathematics, 25.12.2020 04:20


History, 25.12.2020 04:20

Medicine, 25.12.2020 04:20


English, 25.12.2020 04:20

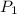 = 100 mm Hg or
= 100 mm Hg or 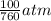 = 0.13157 atm
= 0.13157 atm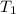 =
= 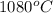 = (1080 + 273) K = 1357 K
= (1080 + 273) K = 1357 K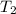 =
= 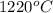 = (1220 + 273) K = 1493 K
= (1220 + 273) K = 1493 K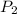 = 600 mm Hg or
= 600 mm Hg or 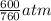 = 0.7895 atm
= 0.7895 atm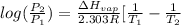
![log(\frac{0.7895}{0.13157}) = \frac{\Delta H_{vap}}{2.303 \times 8.314 J/mol K}[\frac{1}{1357 K} - \frac{1}{1493 K}]](/tpl/images/0231/2242/21278.png)
![log (6) = \frac{\Delta H_{vap}}{19.147}[\frac{(1493 - 1357) K}{1493 K \times 1357 K}]](/tpl/images/0231/2242/46d38.png)
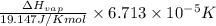
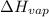 =
= 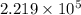 J/mol
J/mol