

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is the cellular process that releases the energy stored in food molecules
Answers: 3

Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1
You know the right answer?
Using the bond energies below, calculate an estimate of ahrxn for the gas phase reaction: qx3 + 3h2...
Questions







Mathematics, 24.09.2019 22:40


Mathematics, 24.09.2019 22:40

Mathematics, 24.09.2019 22:40

Biology, 24.09.2019 22:40

Mathematics, 24.09.2019 22:40


History, 24.09.2019 22:40



Mathematics, 24.09.2019 22:40

Physics, 24.09.2019 22:40

Mathematics, 24.09.2019 22:40

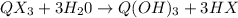
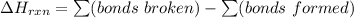
![\Delta H_{rxn}=[3(Q-X)+6(O-H)]-[3(Q-O)+3(H-X)]](/tpl/images/0231/1975/1f975.png)
![\Delta H_{rxn}=[3(240)+6(464)]-[3(359)+3(449)]=1080kJ/mol](/tpl/images/0231/1975/be65c.png)


