
Chemistry, 14.09.2019 10:10 mwms2018sg
You have a 0.5 l of a 1.0 m buffer solution with pka = 5.14 and current ph = 5.23. calculate the new ph when 1.50 ml of 5.00 m hcl is added. be mindful of units. (how can you tell if your answer makes sense? ) ph = pka + 109 u base 5.23 - 5.14+ log ( )

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

You know the right answer?
You have a 0.5 l of a 1.0 m buffer solution with pka = 5.14 and current ph = 5.23. calculate the new...
Questions

English, 03.04.2020 03:08




Mathematics, 03.04.2020 03:09


Mathematics, 03.04.2020 03:09








Mathematics, 03.04.2020 03:09




Mathematics, 03.04.2020 03:09

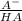
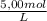 ÷ 0,5 L = 0,015 M of HCl
÷ 0,5 L = 0,015 M of HCl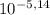
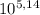 =
= ![\frac{[0,015M -x][0,55M-x]}{[0,45M-x]}](/tpl/images/0231/2479/0fe82.png)


