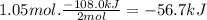Chemistry, 14.09.2019 10:20 coontcakes
the decomposition of hydrogen peroxide, h2o2, has been used to provide thrust in the control jets of various space vehicles. using the supplemental data, determine how much heat (in kj) is produced by the decomposition of 1.05 mol of h2o2 under standard conditions.
2 h2o2(l) → 2 h2o(g) + o2(g)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1


Chemistry, 23.06.2019 06:10
How can liquids be seperated by density a the liquids are absorbed onto a paper b the liquids are turned into seperate vapors c the liquids are collected as they evaporate d the liquids are allowed to seperate into layers
Answers: 1

Chemistry, 23.06.2019 07:30
Using this reversible reaction, answer the questions below: n2o4 2no2 (colorless) (reddish-brown) -as the temperature increased, what happened to the n2o4 concentration? -was the formation of reactants or products favored by the addition of heat? -which reaction is exothermic? right to left or left to right? -if the change of enthalpy of this reaction when proceeding left to right is 14 kcal, which chemical equation is correct? n2o4 2no2 + 14 kcal n2o4 2no2, hr = +14 kcal n2o4 + 14 kcal 2no2 n2o4 2no2, hr = -14 kcal
Answers: 1
You know the right answer?
the decomposition of hydrogen peroxide, h2o2, has been used to provide thrust in the control jets of...
Questions

Physics, 07.10.2021 19:40

Social Studies, 07.10.2021 19:40




Mathematics, 07.10.2021 19:40


Mathematics, 07.10.2021 19:40


Mathematics, 07.10.2021 19:40


Social Studies, 07.10.2021 19:40


English, 07.10.2021 19:40

Medicine, 07.10.2021 19:40


Mathematics, 07.10.2021 19:40

Chemistry, 07.10.2021 19:40


Computers and Technology, 07.10.2021 19:40