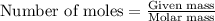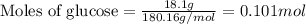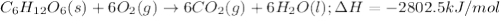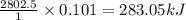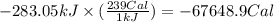Chemistry, 14.09.2019 10:20 GalaxyCraft4991
the oxidation of the sugar glucose, c6h12o6, is described by the following equation. c6h12o6(s) + 6 o2(g) → 6 co2(g) + 6 h2o(l) δh = −2802.5 kj/mol the metabolism of glucose gives the same products, although the glucose reacts with oxygen in a series of steps in the body.
(a) how much heat in kilojoules can be produced by the metabolism of 18.1 g of glucose?
(b) how many calories can be produced by the metabolism of 18.1 g of glucose?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2


You know the right answer?
the oxidation of the sugar glucose, c6h12o6, is described by the following equation. c6h12o6(s) + 6...
Questions



Mathematics, 07.07.2019 23:00


Mathematics, 07.07.2019 23:00


Computers and Technology, 07.07.2019 23:00

Mathematics, 07.07.2019 23:00

Spanish, 07.07.2019 23:00


Health, 07.07.2019 23:00




Geography, 07.07.2019 23:00




Health, 07.07.2019 23:00

Biology, 07.07.2019 23:00