
You want to determine the density of a compound but have only tiny crystal, and it would be difficult to measure mass and volume accurately. there is another way to determine density, however called the flotation method. if you placed the crystal in a liquid whose density is precisely that of the substance, it would be suspended in the liquid, neither sinking to the bottom of the beaker nor floating to the surface. however, for such an experiment, you would need to have a liquid with the precise density of the crystal. you can accomplish this by mixing two liquids of different densities to create a liquid having the desired density a a consider the following: you mix 7.30 ml of chci3 (d = 1.492 g/ml) and 8.90 ml of chbt3 (d = 2.890 g/ml) giving 16.2 ml of solution. what is the density of this mixture? density = g/ml

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
You want to determine the density of a compound but have only tiny crystal, and it would be difficul...
Questions



Biology, 31.08.2019 10:00


English, 31.08.2019 10:00



Mathematics, 31.08.2019 10:00

Biology, 31.08.2019 10:00

Physics, 31.08.2019 10:00

History, 31.08.2019 10:00

History, 31.08.2019 10:00

Biology, 31.08.2019 10:00

English, 31.08.2019 10:00


Mathematics, 31.08.2019 10:00



History, 31.08.2019 10:00

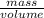
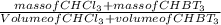
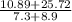 = 2.26g/mL
= 2.26g/mL

